 WELCOME
|
Arturo Manlio Terrés Speziale MDClinical Pathology & Laboratory Medicineaterres@qualitat.ccBorn in Mexico City. February 20, 1953.
Graduated at the Faculty of Medicine UNAM. Revalidated by the Educational Commission for Foreign Medical Graduates. Philadelphia USA. Specialty in Clinical Pathology and Laboratory Medicine. CMN-IMSS-UNAM. Certificate by Clinical Pathology National Board. Postgraduate thesis dealt on Septicaemia, Blood Culture and Mechanisms of Resistance to Antibiotics. Revalidated Postgraduate studies by National Board of Medical Examiners. Philadelphia USA. Training Courses at Max Finland Institute, Boston Massachusetts, Mayo Clinic, Rochester Minnesota and Baylor University Medical Centre Dallas, Texas. Including Diplomas on Strategic Management of Health Services, Quality Assurance and Accreditation (JCAH), Advanced Six Sigma Biostatistics, Reengineering, Analytical Quality Control, Informatics and Telematics, among other.
Founder of the Clinical Laboratory and the Blood Bank as well as the Specialty and the Pathology Division at The American British Cowdray Hospital, recognized by COMPAC & UNAM. Former Director and Advisor of several National Clinical Laboratories including Hospitals, Reference Laboratories and Private Laboratories as well as Research and Development Advisor of several Transnational Companies of Diagnostics and Health Informatics. Leading member of the Coordinating Group the Mexican Program for Certification of Clinical Laboratories that gave rise to the Official Mexican Standard NOM-166. Collaborator on "Quality Improvement of Clinical Laboratories of Latin America" project developed by AMBC-IFCC-COLABIOCLI-PAHO.
Member of the Technology Committee of NCCLS-USA. Has represented WASPaLM at PAHO collaborating in the promotion of het Ethics Code for Clinical Laboratories of Latin America. Coordinator of the Quality Improvement Program for Clinical Laboratories in Latin America to which gave rise to PROMECAL GUIDE 2013:001 of ALAPAC / ML.
Awarded with the National Research Prize "Dr. Luis Rodríguez Villa "in 1991, 1994,1997. Author and Co-Author of three books and more than one hundred scientific articles published in national and international medical journals. Editor of Anales Médicos del Hospital ABC 1989-1992. Co-Editor of the Latin American Journal of Clinical Pathology. Has lectured innumerable courses and conferences inside and outside the Mexico.
Currently holds the position of General Director & Chief Executive Officer of the External Quality Assessment Scheme www.qualitat.cc recognized by the Mexican Accreditation Entity AC on ISO /IEC 17043: 2023 Standard, serving 1,500 laboratories through 6,000 programs throughout Mexico. ARTURO TERRES MD CURRICULUM VITAE < >
QUALITY IMPROVEMENT PROGRAMBIOETHICS AND MEDICAL RELEVANCE FOR CLINICAL LABORATORIESPROMECAL IS A PROBONO: Educational, Non Profit, Voluntary Program for Clinical Laboratories willingto participate, solving the inherent problem of unaffordable costs of Accreditation and Certification Programs.The central feature of this program is that it provides sustention to be treated on the basis of a principle ofsuitability and competition, in order to be carried out exclusively among peer connoisseurs,between authentic Clinical Laboratory Professionals.. DESCRIPTION. PROMECAL arises on the principles of bioethical and medical relevance with extensive knowledge andexperience on Clinical Laboratory Science, providing two basic tools.1. 2013.001 GUIDELINE: Guide for Clinical Laboratory Quality: Bioethics and Medical Relevance.2. 2013.001 CHECKLIST: Audit For Analysis Of Clinical Laboratories, Bioethics, Quality, and Medical Relevance.. SCOPE AND PURPOSE. The Guideline for Clinical Laboratory: Bioethics, Quality and Medical Relevance has greatimportance since it summarizes the specifications that must be met for the organization and operation of theMedical Laboratories under PROMECAL. The Guide was developed based on five basic references, fivepremises, and an accurate glossary.FUNDAMENTS 1. MEDICAL RELEVANCE2. FITNESS3. DICHOTOMY4. AUDIT5. RECOGNITION. PROMECAL has this internet support where you will find severalfreely available tools including:
1. AUDIT: STRUCTURE, PROCESS AND PERFORMANCE2. MEDICAL RELEVANCE EXERCISES3. DIPLOMA ON BIOETHICS, QUALITY AND MEDICAL RELEVANCE4. IMPROVEMENT PLAN. CONCLUSION. Our Mission is to collaborate with Clinical Laboratory Professionals reliably; detecting and exploitingimprovement opportunities, providing a comprehensive system including planning, organizationdevelopment and control through training Programs, counseling and assistance using the latesttechnology with effectiveness in order to be capable to continually improve the qualityand opportunity in decision-making, to ensure that participants report reliable results thatminimize risks and costs for the benefit of their patients and customers.
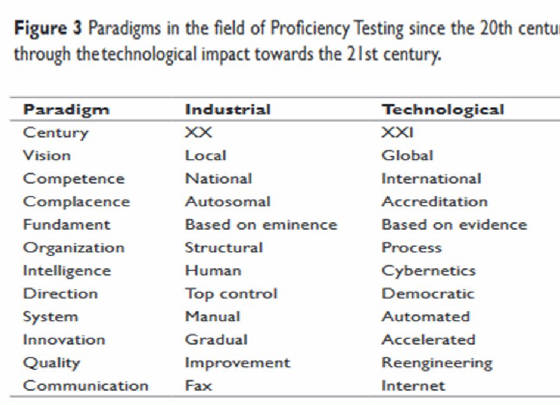
Terres-Speziale AM.Baylor University Medical Center Proceedings. 1989 < > Terres-Speziale AM.Int.J.Clin.Path.2020 < > Terres-Speziale AM.Int.J.Clin.Path.2022 < > Terres-Speziale AM. Int.J.Clin.Path.2023 < >
|





