
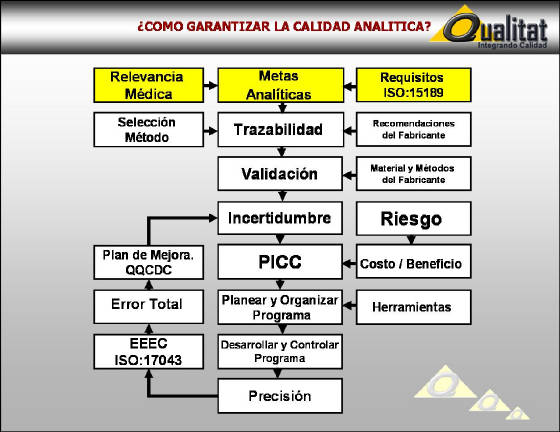
METAS
ANALITICAS CON RELEVANCIA MEDICA


CC = CONTROL DE CALIDAD
CVA = COEFICIENTE DE VARIACION ANALITICO %
CVB = COEFICIENTE DE VARIACION BIOLOGICA %
CVR
= COEFICIENTE DE VARIACION RELATIVO
CC = CVA < CVB
CVR = CVA / CVR
CC
= CVR < 1.0
4 Sigma: Tonks: CVR < 0.250
5 Sigma: Aspen: CVR < 0.125
6
Sigma: CVR < 0.04
SIGMA 4 = META ANALITICA EEEC = ESQUEMA EVALUACION EXTERNA DE
LA CALIDAD
SIGMA 5 = META ANALITICA
PICC = PROGRAMA INTERNO DE CONTROL DE CALIDAD
TRABAJOS Y CONCLUSIONES DEL CONSENSO DE ESTOCOLMO 2015
Defining analytical performance specifications: Consensus Statement from
the 1st Strategic
Conference of the European Federation of Clinical Chemistry and Laboratory Medicine 2015
Report from the first EFLM Strategic
Conference. By Maria Stella Graziani Chair of the EFLM Communication Committee. Clin Lab Med 2015.
Sverre
Sandberg: Defining analytical performance specifications: Consensus Statement from the 1st Strategic Conference of the European
Federation of Clinical Chemistry and Laboratory Medicine. Clin Lab
Med 2015.
Geir Thue, Sverre Sandberg.
Analytical performance specifications based on how clinicians use laboratory tests. Experiences from a post-analytical external
quality assessment programme Clin Lab Med 2015
Matthias Orth. Are regulation-driven performance criteria still acceptable? – The German point of view. Clin
Lab Med 2015
REVISAR ARTICULOS
1. CLIN LAB MED 2015
2. CLIN LAB MED 2015
3. CLIN LAB MED 2015
4. CLIN LAB MED 2015
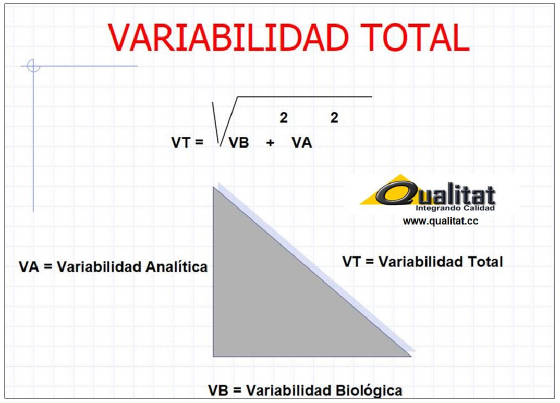
METAS ANALITICAS CON RELEVANCIA MEDICA
DESARROLLADAS SOBRE LA BASE DE
LA VARIABILIDAD BIOLOGICA
Establecer
metas analíticas específicas, medibles, alcanzables y retadoras es el primer paso en cualquier sistema de control
de calidad.
En el laboratorio clínico, al calcular el coeficiente de
variación relativo podemos definir fácilmente las metas de cualquier analito con la
única condición de que se cuente con límites de referencia adecuados para la
población atendida y se conozca el coeficiente de
variación
analítico de la prueba (CV%). La mejora de la calidad y el avance en el logro de los indicadores depende en gran medida de establecer
el nivel actual en el que se encuentra el laboratorio en cada una de las pruebas que procesa ya
que a partir de ahí se puede desarrollar
la mejora
de las prácticas y elevar el nivel de la confiabilidad del laboratorio.
El
logro de las metas analíticas depende del nivel en el que se apliquen. En el Programa Interno de Control de Calidad
dependen en gran
medida del nivel tecnológico con el que se cuente. En los Esquemas de Evaluación
Externa de Calidad, la variabilidad es mayor debido a
que los intervalos de confianza varían
en forma inversa al nivel de incertidumbre, dado el número de variables que intervienen en el proceso.
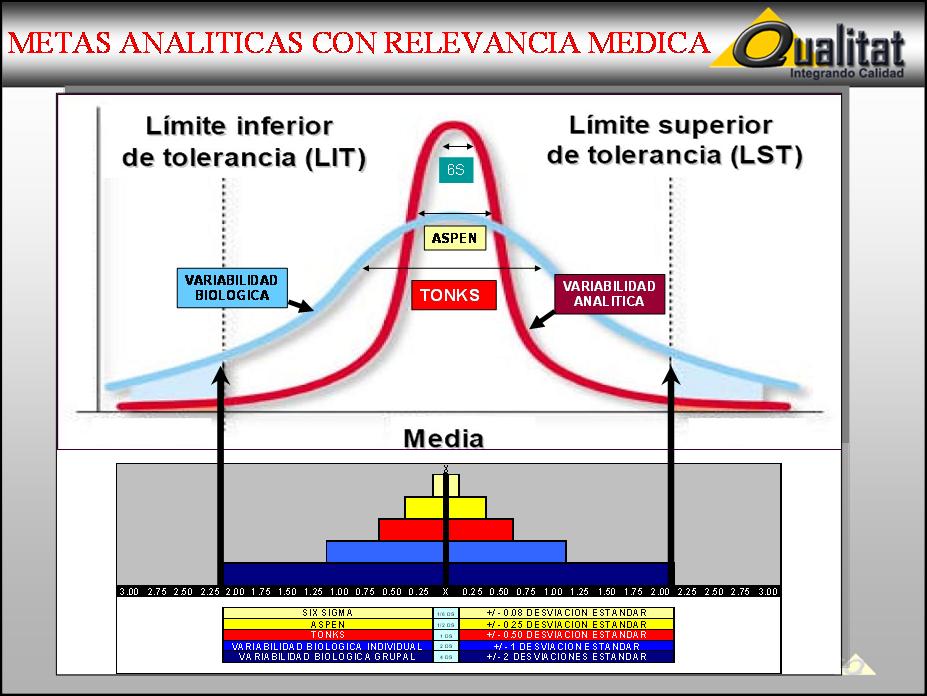
RESUMEN: LA APLICACION DE LAS METAS ANALITICAS SIGMA DEPENDE
DEL NIVEL TECNOLOGICO Y DE NIVEL DE APLICACION
- 4 Sigma : Es una meta adecuada para pruebas manuales. Equivale a 1 DS del rango biológico de referencia
- 5 Sigma : Es una meta adecuada para las
pruebas semiautomatizadas. Equivale a 1/2 DS del mismo rango
- 6 Sigma : Es una meta adecuada para las pruebas automatizadas. Equivale a 1/6
DS del mismo rango.
Las metas analíticas que podemos establecer sobre la base de los niveles sigma son las siguientes.
- 4 Sigma: Nivel Tonks. Confiabilidad 95.0%
- 5 Sigma: Nivel Aspen. Confiabilidad 97.5%
- 6 Sigma: Nivel Six Sigma. Confiabilidad
99.9%
El Nivel Six Sigma equivale al Error Estándar
de la Media cuando la muestra es de 36 elementos.
ESM = DS / RAIZ CUADRADA ( N )
DETERMINACION DE METAS ANALITICAS SIX SIGMA. REV MEX PAT CLIN 2007
DIABETES MELLITUS. METAS ANALITICAS SIX SIGMA. REV MEX PAT CLIN 2008
| | | | | | | | | | | | | |
| BIOMETRIA HEMATICA | UNIDADES |
| MIN | MAX |
| TONKS | ASPEN | 6
SIGMA |
| CLIA; CAP; AAB; NYS |
|
| Eritrocitos | M / uL | | 4.5 | 6.5 | | 9.1% | 4.5% | 1.5% | | 6.0% |
|
| Hemoglobina | g/dL | | 13.5 | 18.0 | | 7.1% | 3.6% | 1.2% | | 7.0% |
|
| Hematocrito | % | | 38.3 | 50.0 | | 6.7% | 3.3% | 1.1% | | 6.0% |
|
| VCM | fL | | 85.0 | 97.5 | | 3.4% | 1.7% | 0.6% | | 2.3% |
|
| CMHc | pg | | 27.0 | 35.0 | | 6.5% | 3.2% | 1.1% | | 2.2% |
|
| CMHb | g/dL | | 28.0 | 37.0 | | 6.9% | 3.5% | 1.2% | | 2.7% |
|
| Leucocitos | mil / uL | | 4.0 | 11.0 | | 23.3% | 11.7% | 3.9% | | 15.0% |
|
| Plaquetas | mil / uL | | 150.0 | 400.0 | | 22.7% | 11.4% | 3.8% | | 25.0% |
|
| | |
| | | | 10.7% | 5.4% | 1.8% |
| 8.3% |
|
| COAGULACION | UNIDADES |
| MIN | MAX |
| TONKS | ASPEN | 6
SIGMA |
| CLIA; CAP; AAB; NYS |
|
| TP | SEGUNDOS | | 9.0 | 15.0 | | 12.5% | 6.3% | 2.1% | | 15.0% |
|
| TP | INR | | 0.5 | 1.5 | | 25.0% | 12.5% | 4.2% | | 15.0% |
|
| TTP | SEGUNDOS | | 15.0 | 45.0 | | 25.0% | 12.5% | 4.2% | | 15.0% |
|
| TT | SEGUNDOS | | 5.0 | 15.0 | | 25.0% | 12.5% | 4.2% | | 15.0% |
|
| AT III | % | | 75.0 | 125.0 | | 12.5% | 6.3% | 2.1% | | 8.3% |
|
| Fibrinógeno | mg / dL | | 200.0 | 400.0 | | 16.7% | 8.3% | 2.8% | | 20.0% |
|
| | |
| | | | 19.4% | 9.7% | 3.2% |
| 14.7% |
|
| BIOQUIMICA | UNIDADES |
| MIN | MAX |
| TONKS | ASPEN | 6
SIGMA |
| CLIA; CAP; AAB; NYS |
|
| Osmolalidad | mOsm/L | | 285.0 | 310.0 | | 2.1% | 1.1% | 0.4% | |
|
|
| Glucosa | mg/dL | | 60.0 | 100.0 | | 12.5% | 5.6% | 1.9% | | 10.0% |
|
| Nitrógeno de Urea (BUN) | mg/dL | | 6.0 | 20.0 | | 26.9% | 13.5% | 4.5% | | 9.0% |
|
| Creatinina | mg/dL | | 0.5 | 1.2 | | 20.6% | 10.3% | 3.4% | | 15.0% |
|
| Acido Úrico | mg/dL | | 2.4 | 7.0 | | 24.5% | 12.2% | 4.1% | | 17.0% |
|
| Colesterol Total | mg/dL | | 100.0 | 200.0 | | 16.7% | 8.3% | 2.8% | | 10.0% |
|
| Colesterol HDL | mg/dL | | 15.0 | 55.0 | | 28.6% | 14.3% | 4.8% | | 30.0% |
|
| Colesterol LDL | mg/dL | | 50.0 | 130.0 | | 22.2% | 11.1% | 3.7% | |
|
|
| Triglicéridos | mg/dL | | 50.0 | 150.0 | | 25.0% | 12.5% | 4.2% | | 25.0% |
|
| Bilirrubinas Totales | mg/dL | | 0.1 | 1.0 | | 40.9% | 20.5% | 6.8% | | 20.0% |
|
| Bilirrubinas Directa | mg/dL | | 0.0 | 1.0 | | 49.0% | 24.5% | 8.2% | |
|
|
| Bilirrubina Indirecta | mg/dL | | 0.0 | 1.0 | | 49.0% | 24.5% | 8.2% | |
|
|
| Proteínas Totales | g/dL | | 6.4 | 8.8 | | 7.9% | 3.9% | 1.3% | | 10.0% |
|
| Albúmina | g/dL | | 3.4 | 4.8 | | 8.5% | 4.3% | 1.4% | | 10.0% |
|
| ALT ( TGP ) | U/L | | 2.5 | 50.3 | | 45.2% | 22.6% | 7.5% | | 20.0% |
|
| AST ( TGO ) | U/L | | 2.2 | 43.7 | | 45.2% | 22.6% | 7.5% | | 20.0% |
|
| DHL | U/L | | 17.8 | 356.9 | | 45.2% | 22.6% | 7.5% | | 20.0% |
|
| Fosfatasa Alcalina | U/L | | 5.8 | 116.4 | | 45.2% | 22.6% | 7.5% | | 30.0% |
|
| GGT | U/L | | 2.8 | 56.4 | | 45.2% | 22.6% | 7.5% | | 30.0% |
|
| Amilasa | U/L | | 6.1 | 122.0 | | 45.2% | 22.6% | 7.5% | | 30.0% |
|
| Lipasa | U/L | | 9.0 | 180.3 | | 45.2% | 22.6% | 7.5% | | 30.0% |
|
| CK | U/L | | 12.5 | 250.0 | | 45.2% | 22.6% | 7.5% | | 30.0% |
|
| MB | U/L | | 1.0 | 20.0 | | 45.2% | 22.6% | 7.5% | | 3 DS |
|
| Sodio | mEq/L | | 130.0 | 145.0 | | 2.7% | 1.4% | 0.5% | | 0.9% |
|
| Potasio | mEq/L | | 3.3 | 5.5 | | 12.5% | 6.3% | 2.1% | | 5.0% |
|
| Cloro | mEq/L | | 91.0 | 107.0 | | 4.0% | 2.0% | 0.7% | | 5.0% |
|
| CO2 | mEq/L | | 20.0 | 30.0 | | 10.0% | 5.0% | 1.7% | | 8.0% |
|
| Calcio | mg/dL | | 8.2 | 10.2 | | 5.4% | 2.7% | 0.9% | | 8.0% |
|
| Fósforo | mg/dL | | 2.7 | 4.5 | | 12.5% | 6.3% | 2.1% | | 12.0% |
|
| Magnesio | mg/dL | | 1.5 | 3.0 | | 16.7% | 8.3% | 2.8% | | 25.0% |
|
| Hierro | ug/dL | | 37.0 | 158.0 | | 31.0% | 15.5% | 5.2% | | 20.0% |
|
| | |
| | | | 27.0% | 13.5% | 4.5% |
| 17.3% |
|
| INMUNOPROTEINAS | UNIDADES |
| MIN | MAX |
| TONKS | ASPEN | 6
SIGMA |
| CLIA; CAP; AAB; NYS |
|
| IGG | mg/dL | | 700.0 | 1600.0 | | 19.6% | 9.8% | 3.3% | | 25.0% |
|
| IGA | mg/dL | | 70.0 | 400.0 | | 35.1% | 17.6% | 5.9% | | 13.5% |
|
| IGM | mg/dL | | 40.0 | 230.0 | | 35.2% | 17.6% | 5.9% | | 16.8% |
|
| IGE | UI/ml | | 1.0 | 100.0 | | 49.0% | 24.5% | 8.2% | | 20.0% |
|
| C3 | mg/dL | | 90.0 | 180.0 | | 16.7% | 8.3% | 2.8% | | 8.4% |
|
| C4 | mg/dL | | 10.0 | 40.0 | | 30.0% | 15.0% | 5.0% | | 16.0% |
|
| AEL | UI/ml | | 1.0 | 200.0 | | 49.5% | 24.8% | 8.3% | | 38.5% |
|
| FR | UI/ml | | 1.0 | 30.0 | | 46.8% | 23.4% | 7.8% | | 13.5% |
|
| PCR | mg/L | | 1.0 | 3.0 | | 25.0% | 12.5% | 4.2% | | 68.3% |
|
| | |
| | | | 30.0% | 15.0% | 5.0% |
| 19.7% |
|
| ENDOCRINOLOGIA | UNIDADES |
| MIN | MAX |
| TONKS | ASPEN | 6
SIGMA |
| CLIA; CAP; AAB; NYS |
|
| CORTISOL ( matinal ) | ug/dL | | 3.8 | 25.0 | | 36.8% | 18.4% | 6.1% | | 25.0% |
|
| INSULINA ( basal en ayuno ) | uU/ml | | 2.6 | 24.9 | | 40.5% | 20.3% | 6.8% | | 25.0% |
|
| FSH ( medio ciclo ) | mU/mL | | 4.7 | 21.5 | | 32.1% | 16.0% | 5.3% | | 25.0% |
|
| PROLACTINA ( sexo femenino ) | ng/mL | | 6.0 | 29.9 | | 33.3% | 16.6% | 5.5% | | 25.0% |
|
| LH ( medio ciclo ) | mU/mL | | 14.0 | 96.0 | | 37.3% | 18.6% | 6.2% | | 25.0% |
|
| TSH | uU/mL | | 0.3 | 3.4 | | 41.9% | 20.9% | 7.0% | | 15.0% |
|
| T3T | ng/dL | | 50.0 | 260.0 | | 33.9% | 16.9% | 5.6% | | 20.0% |
|
| T4T | ug/dL | | 5.1 | 14.1 | | 23.4% | 11.7% | 3.9% | | 20.0% |
|
| FT3 | pg/mL | | 1.4 | 5.5 | | 29.7% | 14.9% | 5.0% | | 30.0% |
|
| FT4 | ng/dL | | 0.3 | 2.5 | | 40.9% | 20.5% | 6.8% | | 10.0% |
|
| ESTRADIOL ( climaterio) | pg/mL | | 8.6 | 49.8 | | 35.3% | 17.6% | 5.9% | | 20.0% |
|
| PROGESTERONA ( fase lutea media ) | ng/mL | | 0.7 | 27.0 | | 47.5% | 23.7% | 7.9% | | 25.0% |
|
| TESTOSTERONA ( adulto masculino ) | ng/dL | | 10.0 | 120.0 | | 42.3% | 21.2% | 7.1% | | 30.0% |
|
| | |
| | | | 36.5% | 18.3% | 6.1% |
| 22.7% |
|
| MARCADORES TUMORALES | UNIDADES |
| MIN | MAX |
| TONKS | ASPEN | 6
SIGMA |
| CLIA; CAP; AAB; NYS |
|
| AFP | ng/mL | | 0.1 | 7.0 | | 48.6% | 24.3% | 8.1% | | 20.0% |
|
| BHGC | mUI/mL | | 0.1 | 5.0 | | 48.0% | 24.0% | 8.0% | | 3 DS |
|
| CA 125 | U/mL | | 0.1 | 35.0 | | 49.7% | 24.9% | 8.3% | | 30.0% |
|
| CA 15-3 | U/mL | | 0.1 | 27.0 | | 49.6% | 24.8% | 8.3% | | 15.5% |
|
| CA 19-9 | U/mL | | 0.1 | 37.0 | | 49.7% | 24.9% | 8.3% | | 44.0% |
|
| CEA | ng/mL | | 0.1 | 3.4 | | 47.1% | 23.6% | 7.9% | | 30.0% |
|
| PSA TOTAL | ng/mL | | 0.1 | 4.0 | | 47.6% | 23.8% | 7.9% | | 30.0% |
|
| PSA LIBRE | ng/mL | | 0.1 | 1.0 | | 40.9% | 20.5% | 6.8% | | 30.0% |
|
| | |
| | | | 47.7% | 23.8% | 7.9% |
| 28.5% |
|
| TORCH | UNIDADES |
| MIN | MAX |
| TONKS | ASPEN | 6
SIGMA |
| CLIA; CAP; AAB; NYS |
|
| Toxoplasma IgG | Cualitativa | | 0.1 | 1.0 | | 40.9% | 20.5% | 6.8% | | POS / NEG |
|
| Toxoplasma IgM | Cualitativa | | 0.1 | 1.0 | | 40.9% | 20.5% | 6.8% | | POS / NEG |
|
| Rubeola IgG | Cualitativa | | 0.1 | 1.0 | | 40.9% | 20.5% | 6.8% | | POS / NEG |
|
| Rubeola IgM | Cualitativa | | 0.1 | 1.0 | | 40.9% | 20.5% | 6.8% | | POS / NEG |
|
| CMV IgG | Cualitativa | | 0.1 | 1.0 | | 40.9% | 20.5% | 6.8% | | POS / NEG |
|
| CMV IgM | Cualitativa | | 0.1 | 1.0 | | 40.9% | 20.5% | 6.8% | | POS / NEG |
|
| Herpes 1 IgG | Cualitativa | | 0.1 | 1.0 | | 40.9% | 20.5% | 6.8% | | POS / NEG |
|
| Herpes 1 Igm | Cualitativa | | 0.1 | 1.0 | | 40.9% | 20.5% | 6.8% | | POS / NEG |
|
| Herpes 2 IgG | Cualitativa | | 0.1 | 1.0 | | 40.9% | 20.5% | 6.8% | | POS / NEG |
|
| Herpes 2 IgM | Cualitativa | | 0.1 | 1.0 | | 40.9% | 20.5% | 6.8% | | POS / NEG |
|
| | |
| | | | 40.9% | 20.5% | 6.8% |
|
|
|
| MONITOR DE DROGAS TX | UNIDADES |
| MIN | MAX |
| TONKS | ASPEN | 6
SIGMA |
| CLIA; CAP; AAB; NYS |
|
| Acetaminofen | ug/mL | | 0.1 | 12.5 | | 49.2% | 24.6% | 8.2% | | 25.0% |
|
| Carbamacepina | ug/mL | | 0.1 | 2.2 | | 45.7% | 22.8% | 7.6% | | 25.0% |
|
| Digoxina | ng/mL | | 0.1 | 1.1 | | 41.7% | 20.8% | 6.9% | | 20.0% |
|
| Fenitoina | ug/mL | | 0.1 | 9.6 | | 49.0% | 24.5% | 8.2% | | 25.0% |
|
| Fenobarbital | ug/mL | | 0.1 | 10.3 | | 49.0% | 24.5% | 8.2% | | 25.0% |
|
| Gentamicina | ug/mL | | 0.1 | 1.4 | | 43.3% | 21.7% | 7.2% | | 25.0% |
|
| Litio | mEq/L | | 0.1 | 0.5 | | 33.3% | 16.7% | 5.6% | | 20.0% |
|
| Salicilato | mg/dL | | 0.1 | 6.5 | | 48.5% | 24.2% | 8.1% | | 20.0% |
|
| Teofilina | ug/mL | | 0.1 | 7.2 | | 48.6% | 24.3% | 8.1% | | 25.0% |
|
| Tobramicina | ug/mL | | 0.1 | 1.8 | | 44.7% | 22.4% | 7.5% | | 25.0% |
|
| Valproico | ug/mL | | 0.1 | 35.5 | | 49.7% | 24.9% | 8.3% | | 25.0% |
|
| Vancomicina | ug/mL | | 0.1 | 6.4 | | 48.5% | 24.2% | 8.1% | | 20.0% |
|
| | |
| | | | 45.9% | 23.0% | 7.7% |
| 23.3% |
|
| NIVELES DE HEMATINICOS | UNIDADES |
| MIN | MAX |
| TONKS | ASPEN | 6
SIGMA |
| CLIA; CAP; AAB; NYS |
|
| Acido Fólico | ug/L | | 2.0 | 20.0 | | 40.9% | 20.5% | 6.8% | | 30.0% |
|
| Vitamina B12 | ng/L | | 190.0 | 765.0 | | 30.1% | 15.1% | 5.0% | | 30.0% |
|
| Ferritina | ug/L | | 20.0 | 120.0 | | 35.7% | 17.9% | 6.0% | | 30.0% |
|
| Hierro | ug/dL | | 37.0 | 158.0 | | 31.0% | 15.5% | 5.2% | | 20.0% |
|
| CTFH | ug/dL | | 270.0 | 360.0 | | 7.1% | 3.6% | 1.2% | | 20.0% |
|
| % Saturación | % | | 20.0 | 50.0 | | 21.4% | 10.7% | 3.6% | | 25.0% |
|
| | |
| | | | 27.7% | 13.9% | 4.6% |
| 25.8% |
|
| DROGAS DE ABUSO EN ORINA | UNIDADES |
| MIN | MAX |
| TONKS | ASPEN | 6
SIGMA |
| CLIA; CAP; AAB; NYS |
|
| Densidad Urinaria | DU | | 1.010 | 1.025 | | 0.4% | 0.2% | 0.1% | | 80.0% |
|
| pH | UI | | 4.8 | 7.4 | | 10.7% | 5.3% | 1.8% | |
|
|
| Creatinina | mg/dL | | 20.0 | 40.0 | | 16.7% | 8.3% | 2.8% | | 15.0% |
|
| Anfetaminas | Cualitativa | | 0.1 | 1.0 | | 40.9% | 20.5% | 6.8% | | POS / NEG |
|
| Barbitúricos | Cualitativa | | 0.1 | 1.0 | | 40.9% | 20.5% | 6.8% | | POS / NEG |
|
| Benzodiacepinas | Cualitativa | | 0.1 | 1.0 | | 40.9% | 20.5% | 6.8% | | POS / NEG |
|
| Canabinoides | Cualitativa | | 0.1 | 1.0 | | 40.9% | 20.5% | 6.8% | | POS / NEG |
|
| Cocaína | Cualitativa | | 0.1 | 1.0 | | 40.9% | 20.5% | 6.8% | | POS / NEG |
|
| Etanol | Cualitativa | | 0.1 | 1.0 | | 40.9% | 20.5% | 6.8% | | 25.0% |
|
| Fenciclidina | Cualitativa | | 0.1 | 1.0 | | 40.9% | 20.5% | 6.8% | | POS / NEG |
|
| LSD | Cualitativa | | 0.1 | 1.0 | | 40.9% | 20.5% | 6.8% | | POS / NEG |
|
| Metacualona | Cualitativa | | 0.1 | 1.0 | | 40.9% | 20.5% | 6.8% | | POS / NEG |
|
| Metadona | Cualitativa | | 0.1 | 1.0 | | 40.9% | 20.5% | 6.8% | | POS / NEG |
|
| Nortriptilina | Cualitativa | | 0.1 | 1.0 | | 40.9% | 20.5% | 6.8% | | POS / NEG |
|
| Opiáceos | Cualitativa | | 0.1 | 1.0 | | 40.9% | 20.5% | 6.8% | | POS / NEG |
|
| Propoxifeno | Cualitativa | | 0.1 | 1.0 | | 40.9% | 20.5% | 6.8% | | POS / NEG |
|
| | |
| | | | 35.0% | 17.5% | 5.8% |
| 40.0% |
|
| URIANALISIS | UNIDADES |
| MIN | MAX |
| TONKS | ASPEN | 6
SIGMA |
| CLIA; CAP; AAB; NYS |
|
| Densidad | DU | | 1.010 | 1.025 | | 0.4% | 0.2% | 0.1% | |
|
|
| PH | UI | | 4.8 | 7.4 | | 10.7% | 5.3% | 1.8% | | 0.04 |
|
| Glucosa | mg/dL | | 0.1 | 30.0 | | 49.7% | 24.8% | 8.3% | | 10.0% |
|
| Cetonas | mg/dL | | 0.1 | 5.0 | | 48.0% | 24.0% | 8.0% | | 25.0% |
|
| Bilirrubinas | mg/dL | | 0.1 | 0.2 | | 16.7% | 8.3% | 2.8% | |
|
|
| Urobilinogeno | mg/dL | | 0.1 | 1.0 | | 40.9% | 20.5% | 6.8% | |
|
|
| Proteinas | mg/dL | | 0.1 | 10.0 | | 49.0% | 24.5% | 8.2% | | 10.0% |
|
| Nitritos | Cualitativa | | 0.1 | 1.0 | | 40.9% | 20.5% | 6.8% | | POS / NEG |
|
| Eritrocitos | Eri / uL | | 0.1 | 5.0 | | 48.0% | 24.0% | 8.0% | |
|
|
| Leucocitos | Leuco / uL | | 0.1 | 10.0 | | 49.0% | 24.5% | 8.2% | |
|
|
| Osmolalidad | mOsm/L | | 400.0 | 800.0 | | 16.7% | 8.3% | 2.8% | |
|
|
| Creatinina | mg/dL | | 20.0 | 40.0 | | 16.7% | 8.3% | 2.8% | | 17.0% |
|
| Microalbúmina | mg/dL | | 0.1 | 10.0 | | 49.0% | 24.5% | 8.2% | | 25.0% |
|
| Hemoglobina | Cualitativa | | 0.1 | 1.0 | | 40.9% | 20.5% | 6.8% | | POS / NEG |
|
| Prueba de embarazo | Cualitativa | | 0.1 | 1.0 | | 40.9% | 20.5% | 6.8% | | POS / NEG |
|
| B-HGC | UI/L | | 0.1 | 10.0 | | 49.0% | 24.5% | 8.2% | | POS / NEG |
|
| Eritrocitos Microscopia | Cualitativa | | 0.1 | 1.0 | | 40.9% | 20.5% | 6.8% | | POS / NEG |
|
| Leucocitos Microscopia | Cualitativa | | 0.1 | 1.0 | | 40.9% | 20.5% | 6.8% | | POS / NEG |
|
| Cilindros Microscopia | Cualitativa | | 0.1 | 1.0 | | 40.9% | 20.5% | 6.8% | | POS / NEG |
|
| Cristales Microscopia | Cualitativa | | 0.1 | 1.0 | | 40.9% | 20.5% | 6.8% | | POS / NEG |
|
| | |
| | | | 36.5% | 18.3% | 6.1% |
| 15.2% |
|
| GASOMETRIA | UNIDADES |
| MIN | MAX |
| TONKS | ASPEN | 6
SIGMA |
| CLIA; CAP; AAB; NYS |
|
| PH | UI | | 7.350 | 7.450 | | 0.3% | 0.2% | 0.1% | | +/- 0.4 |
|
| PO2 | mmHg | | 80.0 | 100.0 | | 5.6% | 2.8% | 0.9% | | 3 DS |
|
| PCO2 | mEq/L | | 35.0 | 45.0 | | 6.3% | 3.1% | 1.0% | | 8.0% |
|
| HCO3 | mEq/L | | 22.0 | 28.0 | | 6.0% | 3.0% | 1.0% | | 10.0% |
|
| CO2 Total | mEq/L | | 23.0 | 30.0 | | 6.6% | 3.3% | 1.1% | | 20.0% |
|
| Saturación O2 | % | | 80.0 | 100.0 | | 5.6% | 2.8% | 0.9% | |
|
|
| Lactato | mmol/L | | 0.4 | 1.2 | | 25.0% | 12.5% | 4.2% | | 30.0% |
|
| Calcio Iónico | mmol/L | | 1.0 | 2.5 | | 21.4% | 10.7% | 3.6% | | 10.0% |
|
| | |
| | | | 9.6% | 4.8% | 1.6% |
| 15.6% |
|
| DETECCION VIRUS | UNIDADES |
| MIN | MAX |
| TONKS | ASPEN | 6
SIGMA |
| CLIA; CAP; AAB; NYS |
|
| Ag de Superficie Hepatitis B | Cualitativa | | 0.1 | 1.0 | | 40.9% | 20.5% | 6.8% | | POS / NEG |
|
| Ac Anti Virus Hepatitis C | Cualitativa | | 0.1 | 1.0 | | 40.9% | 20.5% | 6.8% | | POS / NEG |
|
| Ac Anti Virus HIV 1/2 | Cualitativa | | 0.1 | 1.0 | | 40.9% | 20.5% | 6.8% | | POS / NEG |
|
| Ac Anti HB core | Cualitativa | | 0.1 | 1.0 | | 40.9% | 20.5% | 6.8% | | POS / NEG |
|
| Anti-HAV IgG | Cualitativa | | 0.1 | 1.0 | | 40.9% | 20.5% | 6.8% | | POS / NEG |
|
| Anti-HAV IgM | Cualitativa | | 0.1 | 1.0 | | 40.9% | 20.5% | 6.8% | | POS / NEG |
|
| | |
| | | | 40.9% | 20.5% | 6.8% |
|
|
|
| DIABETES MELLITUS | UNIDADES |
| MIN | MAX |
| TONKS | ASPEN | 6
SIGMA |
| CLIA; CAP; AAB; NYS |
|
| NGSP: HbA1c % | % | | 4.5 | 6.5 | | 9.1% | 4.5% | 1.5% | | 4.4% |
|
| GP3M:
Glucosa Promedio Trimestral | mg/dL | | 75.0 | 135.0 | | 14.3% | 7.1% | 2.4% | |
|
|
| | |
| | | | 11.7% | 5.8% | 1.9% | | 4.4% | |
CLIA
Requirements for Analytical Quality | Federal Register February 28, 1992;57(40):7002-186. |
CLIA Requirements for Analytical Quality | https://www.westgard.com/clia.htm |
TABLA: CLIA , CAP, AAB, NYS -> | http://www.dgrhoads.com/db2004/ae2004.php?B8=+All+&find=&start=1&NOLINKS= |
| | |
| | | |
|
|
|
|
|
|
CALCULADORA SIGMAMETRICS 1.0 < >
CALCULADORA SIGMAMETRICS 2.0 < >










